
CMRR
Center for Magnetic Resonance Research, Department of Radiology
Research Highlights - Neurodegenerative Diseases
You are here
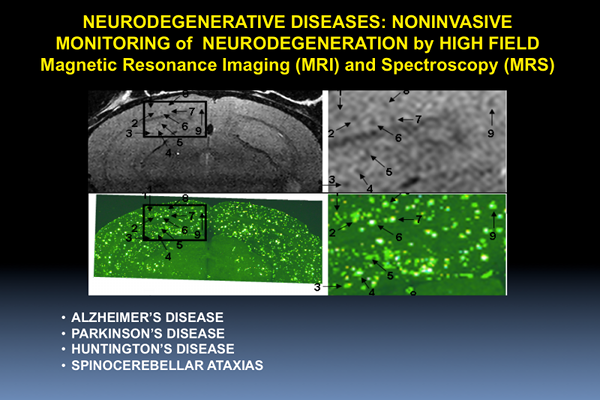
Neurodegenerative Diseases
Noninvasive Monitoring of Neurodegeneration by Magnetic Resonance Imaging (MRI) and Spectroscopy (MRS)
Neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) are neurological disorders in which vulnerable neuron populations in the central nervous system are lost. They are progressive and cause significant physical and psychological burden on individuals, their families, and society. Their pathological progression is particularly difficult to monitor because of the primary location of the problem: the brain. Researchers at CMRR have been developing MR methods to non-invasively monitor structural and functional changes in these brain diseases and together with their colleagues from Departments of Neurology, Neuroscience, Lab Medicine and Pathology and the Institute for Translational Neuroscience they have been validating the developed methods in patients and rodent models of these diseases. Below are highlights of research in this area.
ALZHEIMER’S DISEASE
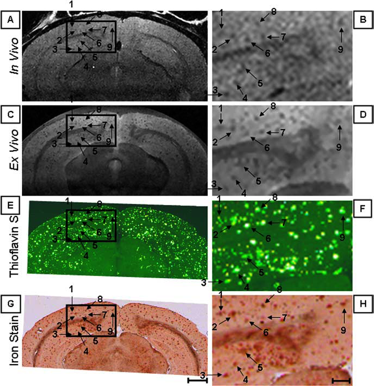
Figure 1. Individual senile plaques can be visualized by MRI in a living Alzheimer’s disease mouse (A, B). The locations of the plaques are confirmed in the same mouse post-mortem by MRI (C, D) and histology (E-H).
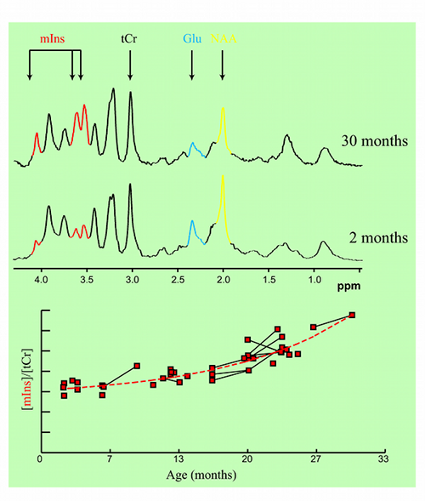
Figure 2. Age-dependent changes in metabolite concentrations observed in living mice.
In collaboration with Mayo Clinic, Rochester, MN, CMRR researchers have focused on the development of methods, identification of magnetic resonance biomarkers, and testing treatment in mouse models. Through the use of novel methods and high field, they were the first to visualize amyloid plaques in living animals using MRI (Figure 1) (1). Additionally, they demonstrated that disease progression can be followed using imaging of amyloid plaques (2). Using imaging techniques developed at CMRR (1,3), they investigated properties of amyloid plaques in different brain regions and the origin of contrast which allows the visualization of plaques in MRI (4). With collaborators at Mayo Clinic, they have developed molecules that enhance visualization of plaques (5). They also detected age-dependent changes in metabolite concentrations in the brains of living mice analogous to those in AD patients using MRS (6). Because of their noninvasive and repeatable nature, these methods could substantially accelerate drug discovery for AD.
PARKINSON’S DISEASE
PD is a degenerative brain disorder associated with loss of dopamine producing brain cells and manifesting with alterations in movement. Cardinal features of PD include asymmetric onset of symptoms such as tremor, slowness, and stiffness. With recent developments in novel MRI methods at the University of Minnesota CMRR researchers are attempting to map structural, functional and biochemical changes that occur in PD. Using a novel high resolution MRI method they were able to detect the asymmetric degeneration in a very small brain structure called the substantia nigra in patients with PD (7,8). Such quantitative mapping techniques are expected to enable tracking disease progression even before patients show motor symptoms (Figure 3). These advancements may further result in a better understanding of the underlying factors that cause PD, and provide a means for monitoring treatments.
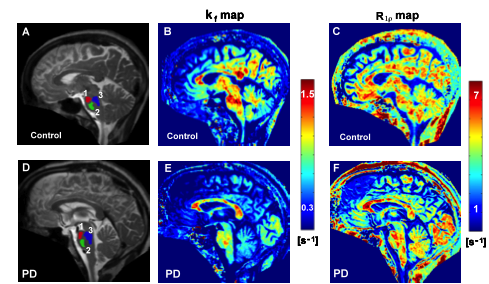
Figure 3. Novel MRI methods demonstrate functional and structural changes in the brainstem of a patient with Parkinson’s Disease relative to a healthy control. The kf map reflects dynamic properties of cellular components while the R1ρ map reflects neuronal integrity. The regions of interest used for analysis are shown on the anatomical images with different colors (1, 2, 3 in A and D).
HUNTINGTON’S DISEASE
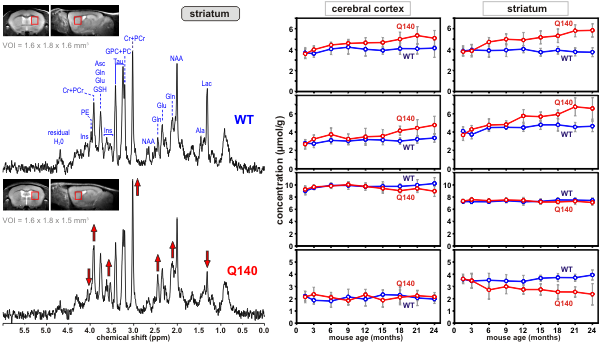
Huntington’s disease (HD) is an inherited neurological disorder that causes progressive loss of motor function, cognitive decline and emotional disturbances. The cascade of events that cause the selective loss of neurons in a brain region called the striatum is not well understood. Transgenic mouse models of HD have emerged as a powerful tool to investigate the pathophysiology of this disease. Using ultra-high field MRS CMRR researchers study the neurochemical changes in transgenic mouse models of HD. Methodology developed at CMRR (9,10) enables reliable quantification of 15 brain metabolites from volumes as small as 4 - 5 microliters (a volume with dimensions of 1-2 mm) (11). This noninvasive method is ideal for investigating neurochemical changes in the progress of HD longitudinally (Figures 4 and 5) and provides insights into the underlying cellular changes in HD.
 |
|
Figure 4. Proton MR spectra obtained from a Huntington’s disease (Q140) and a control mouse (wild type, WT) from the volume-of-interest shown on the images. Differences in peak patterns are visible |
Figure 5. Longitudinal concentration changes in selected metabolites from two brain regions of Huntington’s disease (Q140) and control mice (WT). The divergence of concentrations of some metabolites over time is visible. |
SPINOCEREBELLAR ATAXIAS
Spinocerebellar ataxias (SCAs) are movement disorders that are caused by neurodegeneration in the cerebellum. People who have ataxia have uncontrolled, uncoordinated movements in the way they walk, move their arms or eyes, or talk. Many SCAs are inherited and ultimately fatal. The goal of the ataxia research at CMRR is to identify biological markers that reflect the progressive neuronal dysfunction and loss in the cerebellum and that can be utilized to monitor drug effects in clinical trials. CMRR researchers have shown that many neurochemicals can be reliably quantified by MRS at high and ultra-high fields both in the human (12) and rodent brain (10). Using these methods they have detected similar neurochemical alterations in patients and mouse models that have the same hereditary ataxia (Figure 6) and have shown that the MRS markers are sensitive to progressive neurodegeneration, even before motor symptoms occur (13,14). Therefore these markers can be used in the future to detect cellular changes before irreversible damage to the cerebellum, likely when treatments will be most effective.
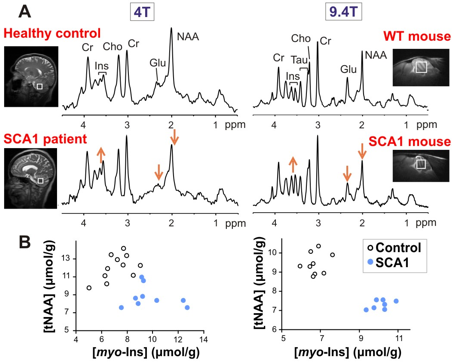
Figure 6. Parallel neurochemical alterations in patients (left) and transgenic mouse models of neurodegeneration (right). (A) Proton MR spectra obtained from the cerebellum of a patient with spinocerebellar ataxia type 1 (SCA1) and a mouse with the same genetic mutation are shown in comparison to healthy controls. (B) Metabolite concentrations obtained from individual subjects distinguish the SCA1 and control groups with no overlap (left: human data, right: mouse data).
REFERENCES
[1] Jack CR, Jr., Garwood M, Wengenack TM, Borowski B, Curran GL, Lin J, Adriany G, Grohn OH, Grimm R, Poduslo JF. In vivo visualization of Alzheimer's amyloid plaques by magnetic resonance imaging in transgenic mice without a contrast agent. Magn Reson Med 2004;52(6):1263-1271.
[2] Jack CR, Jr., Wengenack TM, Reyes DA, Garwood M, Curran GL, Borowski BJ, Lin J, Preboske GM, Holasek SS, Adriany G, Poduslo JF. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer's transgenic mice. J Neurosci 2005;25(43):10041-10048.
[3] Chamberlain R, Reyes D, Curran GL, Marjanska M, Wengenack TM, Poduslo JF, Garwood M, Jack CR, Jr. Comparison of amyloid plaque contrast generated by T2-weighted, T2*-weighted, and susceptibility-weighted imaging methods in transgenic mouse models of Alzheimer's disease. Magn Reson Med 2009;61(5):1158-1164.
[4] Wengenack TM, Reyes DA, Curran GL, Borowski BJ, Lin J, Preboske GM, Holasek SS, Gilles EJ, Chamberlain R, Marjanska M, Jack CR, Jr., Garwood M, Poduslo JF. Regional differences in MRI detection of amyloid plaques in AD transgenic mouse brain. NeuroImage 2011;54(1):113-122.
[5] Poduslo JF, Hultman KL, Curran GL, Preboske GM, Chamberlain R, Marjanska M, Garwood M, Jack CR, Jr., Wengenack TM. Targeting Vascular Amyloid in Arterioles of Alzheimer Disease Transgenic Mice With Amyloid beta Protein Antibody-Coated Nanoparticles. J Neuropathol Exp Neurol 2011;70(8):653-661.
[6] Marjanska M, Curran GL, Wengenack TM, Henry PG, Bliss RL, Poduslo JF, Jack CR, Jr., Ugurbil K, Garwood M. Monitoring disease progression in transgenic mouse models of Alzheimer's disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A 2005;102(33):11906-11910.
[7] Michaeli S, Öz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S, Tuite P. Assessment of brain iron and neuronal integrity in patients with Parkinson's disease using novel MRI contrasts. Mov Disord 2007;22(3):334-340.
[8] Nestrasil I, Michaeli S, Liimatainen T, Rydeen CE, Kotz CM, Nixon JP, Hanson T, Tuite PJ. T1ρ and T2ρ MRI in the evaluation of Parkinson's disease. J Neurol 2010;257(6):964-968.
[9] Tkáč I, Starcuk Z, Choi I-Y, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999;41:649-656.
[10] Pfeuffer J, Tkáč I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J Magn Reson 1999;141(1):104-120.
[11] Tkáč I, Dubinsky JM, Keene CD, Gruetter R, Low WC. Neurochemical changes in Huntington R6/2 mouse striatum detected by in vivo 1H NMR spectroscopy. J Neurochem 2007;100(5):1397-1406.
[12] Tkáč I, Öz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magn Reson Med 2009;62(4):868-879
[13] Öz G, Hutter D, Tkáč I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord 2010;25(9):1253-1261.
[14] Öz G, Nelson CD, Koski DM, Henry PG, Marjanska M, Deelchand DK, Shanley R, Eberly LE, Orr HT, Clark HB. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci 2010;30(10):3831-3838.

