The mission of the core is to provide researchers perfoming clinical research access to the human high-field MR systems located at the Center for MR Research (CMRR). Access is established by providing advice on this new emerging technology and by providing guidance to the possibilities and research opportunities at the CMRR, and to provide assistance in setting up scan protocols, experimental design and conducting some of the studies and consults on safety issues for MR Scans, organizes CPR training for personnel at the CMRR.
Rolf Gruetter, Associate Director, Department of Radiology and Neuroscience, Core
Director
Ivan Tkac, Research Associate, Department of Radiology
Contact us if you have questions or concerns. Or Fairview-University Medical Center Patient Relations at
The MR Core provides access to the following infrastructure at the Center for MR Research:
Glucose analyzer and microfuge
Infusion pumps for administering substances such as glucose, insulin, somatostatin
Fully equipped InVivo Research Patient monitoring system integrated into the 4 Tesla
scanner (end-tidal CO2, pulseoxymetry, ECG, and blood pressure monitoring) and additional
stand-alone pulseoxymeter and blood pressure monitoring devices
The MR Core administeres the following space at the CMRR:
Patient Room (Rm 111E) - providing two full hospital beds, equipped to handle two
simultaneous overnight studies at the Center
Clean Storage Room (Rm 111B) - Storage of monitoring equipment, supplies and linen
Soiled Storage Room (Rm 111G) and ambulance bay (Rm. 111D)
Patient Bathroom (Rm 111H) -
Procedure Room (Rm 117A) - provides the infrastructure to conduct glucose infusions in the
human 7 Tesla and 4 Tesla magnets, houses glucose analyzer, infusion pumps
Physician's Room (Rm 111A) - desk space for clinical collaborators, provides access to the
internet and computing resources at the CMRR
482
508
536
569
593
636
677
678
680
724
Sample publications that resulted from activities of this core:
Gruetter R, Seaquist ER, Ugurbil K (2001) A
mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am
J Physiol Endocrinol Metab 281, E100-12.
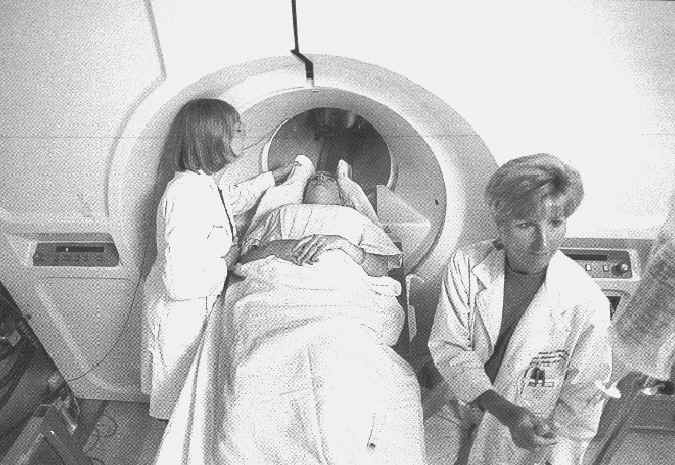
Glucose/insulin clamp procedure in the 4Tesla human system (#482)
Information (click here): How do I sign up for MR Scans ?
Volunteer reimbursement: We need the W-9 [24k] (W-9 instructions [32k]) filled out and signed.
While MR does not contain any known biological risks and thus is one of the safest diagnostic modalities, an MR scan is not without danger. The major danger comes from magnetic objects being introduced into the magnet room or by scanning patients that do not match our inclusion criteria. With appropriate questioning and screening these dangers can be minimized. We therefore ask that you consult with the following two forms:
Potential research subjects, please consult the Screening questionnaire
If you are planning to enter the Center (whether as a subject or a patient or accompanying medical personnel), be sure to strictly adhere to the following safety guidelines and required actions. At all times you must comply with Center personnel instruction while in the building. (Directions to the Center for MR Research)
Upon completion of the scan, you must fill out the Exit form.
Significant risk definition from the CDRH of the FDA
E. Kanal and F.G. Shellock's website
Home page of the Society of MR Technologists (SMRT)
Bill Faulkner's Outsource Inc.
MRI Indications for the referring physician
The basics of MRI, by Hornak