
CMRR
Center for Magnetic Resonance Research, Department of Radiology
Partnership for MRS Biomarker Development
You are here
PROJECT NARRATIVE
The goal of this project is to build a multi-institute partnership to establish the feasibility of using advanced, quantitative imaging technology in the clinical setting for neurodegenerative diseases. The technology is intended for noninvasive monitoring of pathology and effects of treatments in the brain. A high impact is expected in moving potential therapies for a set of movement disorders, spinocerebellar ataxias, and for Alzheimer’s disease from the laboratory to the bedside.
SPECIFIC AIMS
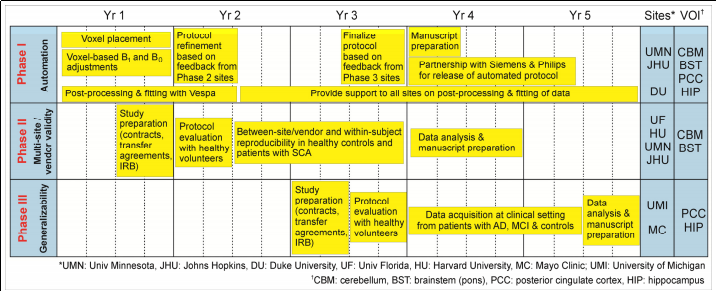
Aim 1: Implement a fully automated advanced MRS acquisition and analysis protocol on Siemens and Philips platforms [University of Minnesota (UMN) - Siemens site; Johns Hopkins University (JHU) - Philips site; Duke University (DU) - analysis site]. The acquisition protocol, including atlas-based voxel positioning will be automated. Spectral processing and fitting will be via extension of the NIH funded, free, open-source Vespa package (Versatile Simulation, Pulses and Analysis).
Aim 2: Establish multi-site/vendor reproducibility of the automated MRS acquisition and analysis protocol in healthy volunteers and patients with SCA [UMN, JHU, Harvard University (HU), University of Florida (UF)]. A standard phantom, healthy volunteers and patients will be scanned to establish within- and between-site reproducibility of neurochemical quantification in the presence of neurodegeneration.
Aim 3: Validate translatability of the advanced MRS protocol to clinical environment and a common neurodegenerative disease [Mayo Clinic]. MRS data will be obtained from patients with AD, mild-cognitive impairment (MCI) and matched controls using the automated protocol by rotating MR technologists in a clinical setting.
ABSTRACT
Clinical trials for neurodegenerative diseases are hampered by the lack of quantitative and objective biomarkers that reflect treatment effects in the brain. Magnetic resonance spectroscopy (MRS) has potential to directly assess disease-modifying effects of therapeutic interventions in the brain. However, MRS has not made the transition to the clinical setting, largely due to lack of standardization of data acquisition and analysis methods and compromised data quality obtained with standard clinical packages, which result in poor reproducibility of neurochemical concentrations. The primary objective of this application is to facilitate translation of advanced MRS technology to the clinical setting in a strategic alliance between MR physicists, software engineers and physician scientists.
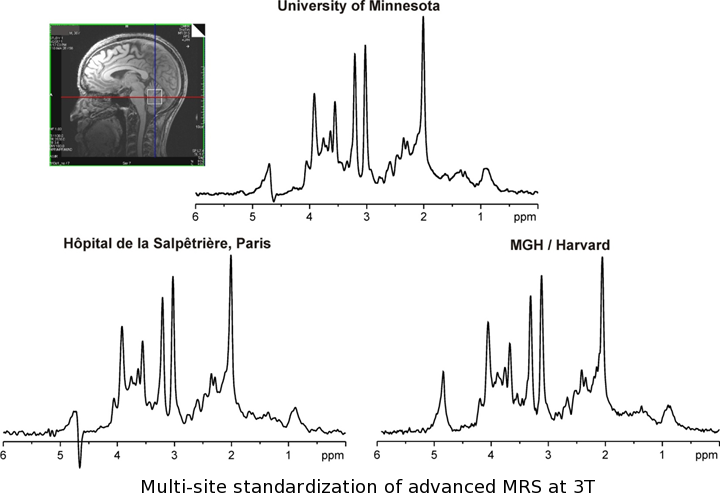
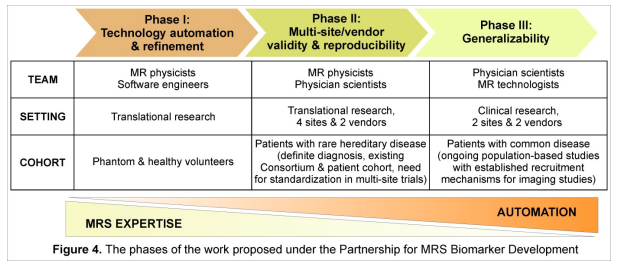
This Partnership for MRS Biomarker Development is comprised of 3 phases and incorporates a gradual shift from MRI/MRS to clinical expertise at the sites involved: Phase I will establish an MR-technologist ready advanced MRS protocol on two widely-used clinical 3T platforms. An optimized semi-LASER (sLASER) sequence was chosen for this implementation because it is considered a top candidate for recommendation for high fields by the MRS Consensus Group. Phase II will assess the performance of the protocol under ideal conditions (efficacy), namely at sites where MRI/S and clinical trial expertise overlap. We focus on hereditary spinocerebellar ataxias (SCA) because the patient cohorts are well-characterized and they present the greatest need for multi-center investigations to sufficiently sample the patient population in trials. Four sites of the Clinical Research Consortium for Spinocerebellar Ataxias (CRC-SCA), which was formed to provide infrastructure for clinical trials in the common SCAs, will participate in this phase. Finally, Phase III will assess the performance of the protocol under ordinary conditions (effectiveness), i.e. in a clinical setting with rotating MR technologists. This phase will focus on Alzheimer disease (AD), the most common cause of age associated cognitive decline and dementia, and take advantage of large ongoing neuroimaging investigations.
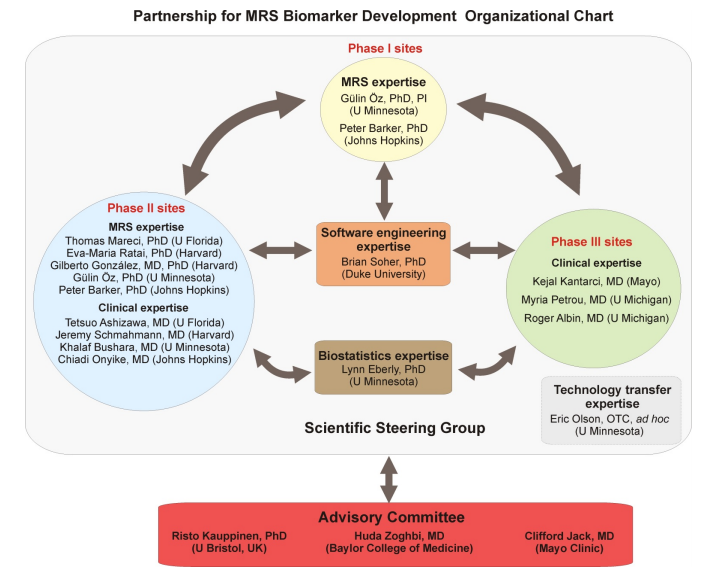
This application has a translational focus. The bioengineering focus areas of this project include advanced high field MRI technology for neuroimaging applications, validation and reproducibility assessment of spectral acquisition and analysis methods and non-invasive technology to assist monitoring treatment response in brain disorders. The partnership sites are the University of Minnesota (leading institution), Johns Hopkins University [Phases I and II], Duke University [Phase I], University of Florida [Phase II], Harvard University [Phase II], Mayo Clinic [Phase III] and University of Michigan [Phase III]. The deliverable of this project will be a turn-key advanced MRS data acquisition and analysis protocol that has been comprehensively evaluated in multiple patient cohorts, brain regions, platforms, and institutions, including the clinical setting. A high impact is expected in early diagnosis and treatment of neurodegenerative diseases.
DEVELOPMENT
Github organization mrs-biomarker-brp: https://github.com/mrs-biomarker-brp
