
CMRR
Center for Magnetic Resonance Research, Department of Radiology
Research Highlights - Body Imaging
You are here
High-Field Body MR Imaging and Spectroscopy
Advantages of ultrahigh magnetic field MRI, due to gains of signal-to-noise-ratio (SNR), parallel imaging performance and novel and/or improved contrast mechanism have been amply demonstrated in the human brain. However, the organ systems and diseases associated with the human torso have been largely excluded from these advances to date due to difficulties that arise when the radiofrequency (RF) wavelength becomes significantly smaller than the object size, as it does in the human torso at 7T. Recent developments, largely coming from our laboratory, demonstrate that these challenges can be overcome.
Our long term goals are to exploit the potential gains available when performing MRI studies at higher magnetic fields to investigate clinical problems in the human torso. To date these efforts have focused developing the hardware, RF management tools, acquisition methods and safety aspects necessary to study several different target organs the human torso including the heart, breasts, kidney, prostate and liver. Greater spatial resolution, spectral resolution and contrast at ultrahigh magnetic fields promises to improve the characterization of disease whether for localizing disease or monitoring progression as in the case of cancer or the diagnosis and assessment of cardiovascular function and health.
TECHNOLOGY DEVELOPMENTS
Coils:
For body applications at high magnetic fields, we have focused on developing RF coils which can be used for both transmission and reception of RF which are closely fitted to the subject’s body. In contrast to transmit RF coils used at lower clinical field strengths where the transmit coils are the size of the scanner bore, these coils have improved transmit efficiency which is needed at these higher field strengths. These smaller transmit coils also help manage electric fields which are responsible for heating in the body.
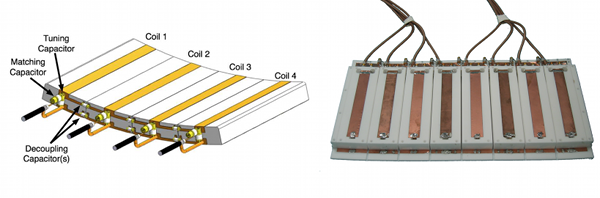
Figure Caption: On the left is the schematic of the stripline/TEM type coil used in a majority of our ultrahigh field body imaging work. On the right, the 8 anterior channels of the actual stripline/TEM RF coil is shown with the protective cover removed. In practice, this plate of coils placed on top of the subject would be paired with a similar array underneath the subject.
RF Management:
With increasing field strength, inhomogeneities in the electromagnetic magnetic fields also increase. The spatial inhomogenetiy is a result of constructive and destructive interferences which occur as the size of the body is larger than the wavelength (~12 cm) at these frequencies (~ 300 MHz). By using multi-channel arrays driven by independent RF amplifiers, many different B1+ shimming strategies have been employed to optimize transmit fields in targeted regions in both head and body applications. The optimization strategy of choice depends on the target region’s size, geometric complexity, and location. In addition, the optimal solution may vary for individual RF pulses within an acquisition sequence.
To address the challenges with transmit B1, we have developed shimming strategies making use of subject dependent calibration scans which can be rapidly acquired.
Movie Caption: Each image shown is acquired by transmitting through one channel at a time, allowing the estimation of channel specific complex B1+ maps. This information can be used to implement RF shimming methods focusing on a wide range of optimization goals.
There is typically a tradeoff between the goals of obtaining B1+ efficiency and homogeneity. This tradeoff plays a critical role at 7T where current limitations in achievable peak B1+ in the body are a result of complex and destructively interfering RF fields, hardware limitations (RF coils, transmit chain efficiency, RF amplifier output) and local SAR (heating) constraints. If possible, it would be ideal to perform all imaging under a homogeneous transmit B1 distribution, but in many instances this is impractical at UHF.
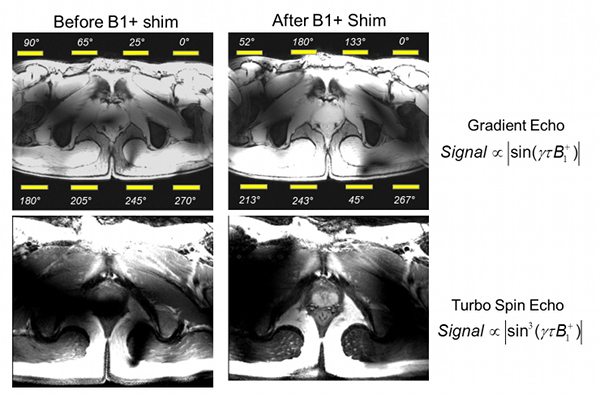
Figure Caption: Shown here is a demonstration of B1+ shimming optimizing for transmit efficiency in the region of the prostate. Using the default transmit phases of the surface array coil (left), the B1+ in the region of the prostate interferes destructively creating a black hole in the image. After optimizing the transmit phases for efficiency in the prostate (right), the expected signal and contrast are achieved in the gradient echo and spin echo images, respectively.
Safety:
To ensure subject safety, model based simulation and real time power monitoring are both required. By simulating the RF coil and shimming conditions used in actual experiments, the limits on how much power we can use can be estimated. The main concern is patient heating which is a result of electric field component of the radiofrequency energy. These electric fields become less homogeneous at higher magnetic fields making local heating the concern rather than global heating which is the focus at lower field strengths.
By developing our own power monitoring setup, each channel of our multi-channel transmit system is monitored and set to turn off if the maximum allowed average power is exceeded. Other work being investigated by our lab is focusing on improved safety guidelines and monitoring based on estimating the temperature in the tissue. Our current safety limits are conservative as they are based the power absorbed by the tissue and do not account for the potential of cooling mechanisms such as perfusion.
APPLICATIONS
Prostate:
There is great potential for the technological developments we are pursuing in our lab to provide quantitative and sensitive MRI markers that better identify prostate cancer and differentiate pathologically indolent from aggressive disease.
We have designed, constructed and validated novel coil designs that result in improved imaging of the prostate at 7T compared to standard clinical field strength systems. Multiple studies have also been investigating the potential for higher spectral and spatial resolution spectroscopic imaging and addressing the challenges of performing dynamic contrast enhanced MRI (DCE-MRI) with standard contrast agents. The use of contrast agents at higher magnetic fields is challenging due to competing contrast mechanisms which do not favor increasing field.
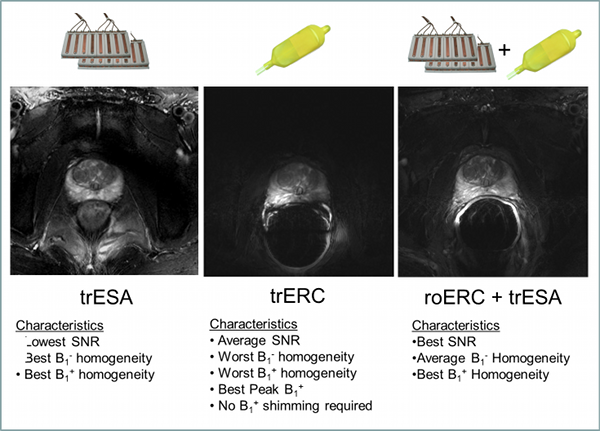
Figure Caption: Axial T2w-TSE images of the prostate with the transmit-receive external surface array (trESA), a transmit-receive endorectal coil (trERC) and a combined trESA and receive-only ERC (trESA+roERC). The horizontal arrow in demonstrates the low SNR and contrast resulting from limited peak B1+. The advantage of the trESA+roERC is improved SNR and transmit B1 homogeneity.
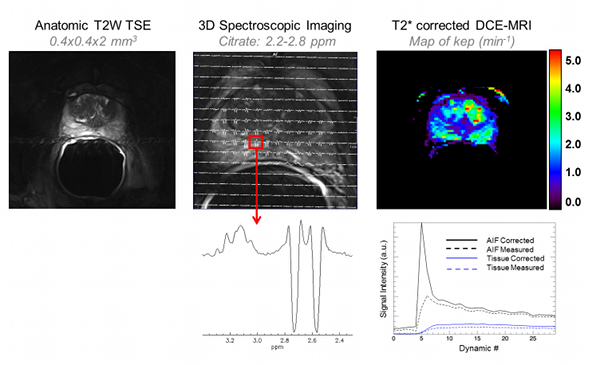
Figure Caption: In additional to high resolution anatomic images, the combined transmit-receive surface array and receive only endorectal coil allow, for the first time, high resolution 3D spectroscopic imaging and dynamic contrast enhanced MRI studies to be performed at 7T in the prostate. Spectroscopy: The greater spectral resolution will allow for improved quantification of individual metabolites including spermine which has been most closely correlated with cancer aggressiveness while the improved spatial resolution will allow smaller volmes of disease to be identified and monitored. DCE-MRI: While T2* effects of typical contrast agents compete with the desired T1 signal enhancement (dashed curves) multi-echo acquisitions can be acquired to correct these effects (solid curves) thus allowing pharmacokinetic modeling in the prostate.
Kidney:
In addition to obtaining high resolution anatomic images of the kidney at 7T, our initial focus in the kidney was to develop the ability to acquire high resolution non-contrast enhanced renal angiography data. The potential advantage of performing these studies at 7T includes increased vessel conspicuity which would benefit a multitude of clinical applications including the evaluation of patients with renal insufficiency or failure, uncontrolled hypertension, and preoperative evaluation of renal vascular anatomy. The subset of patients with renal insufficiency or failure poses a unique clinical challenge in that their renal impairment may preclude the use of gadolinium-based contrast agents due to the possibility of developing nephrogenic systemic fibrosis, a debilitating and potentially fatal disease.
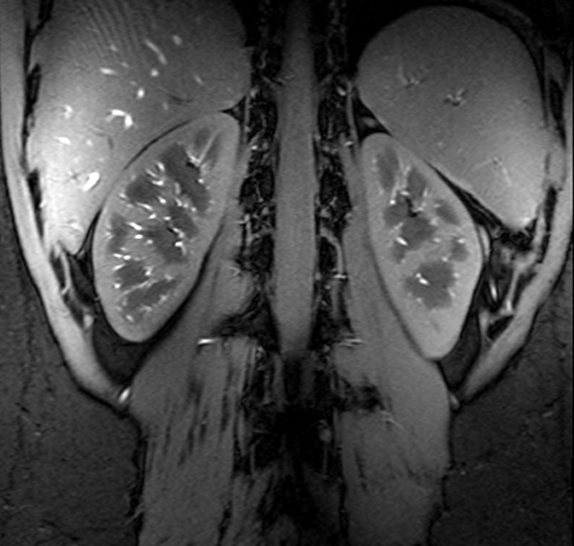
Figure Caption: A high resolution gradient echo anatomic image of the kidneys acquired at 7T using a multiple region B1+ solution optimized for transmit efficiency.
Unlike the high resolution anatomic scans, angiography acquisitions have much higher demands in terms of the RF required. There are many RF pulses in a given repetition of the angiography sequence, each having its own requirements in terms of homogeneity and efficiency given the system and safety limits on RF power. Therefore, multiple B1+ shimming solutions were calculated and dynamically applied during the sequence to obtain high quality renal angiograms without the use of an exogenous contrast agent.
(Kidney7T_volunteerJ_vol_MIP movie)
Movie Caption: A single multi-slice calibration scan, acquired in a single breath hold, was used to calculate multipile B1+ shim solutions which were dynamically applied to achieve these results. An inversion RF pulse to suppress the background parenchyma was optimized more for efficiency because of limitations in peak available RF power and the excitation RF pulses were optimized more for homogeneity to obtain uniform signal across the vessels.
Cardiac - Functional:
Cardiac anatomic and functional imaging can benefit from both increased spatial and temporal resolution at high magnetic fields. The initial goals of our work in the area of functional cardiac imaging included a field comparison between 1.5, 3.0 and 7.0 tesla in collaboration with Oxford University.
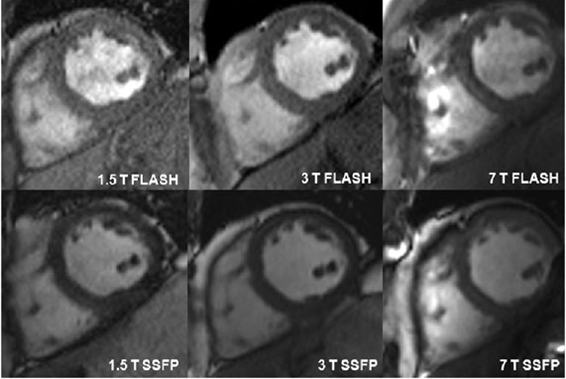
Figure Caption: SSFP and FLASH cine imaging at 7 T is technically feasible and provide valid assessment of cardiac volumes and mass compared with functional cardiac imaging at 1.5 T and 3 T field strengths. A smaller difference in volumes and mass measurements between SSFP and FLASH imaging were observed at 7 T than 1.5 T and 3 T. Signal to noise (SNR) and contrast to noise (CNR) ratios were significantly higher at 7 T than the lower field strengths for both both SSFP and FLASH.
Additional work has focused on the acquisition of high temporal resolution functional acquisitions, volumetric cine acquisitions and real-time functional studies making use of high acceleration factors made possible GRAPPA and T-GRAPPA reconstruction strategies.
Cardiac – Coronary Artery:
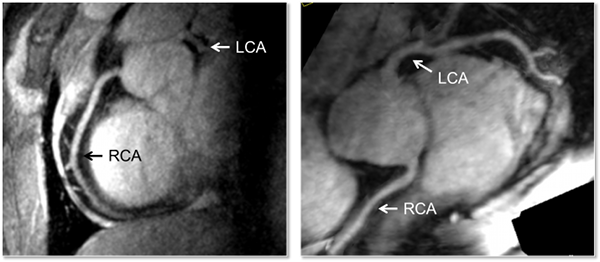
Performing right coronary artery imaging at 7T has been shown to improve the signal to noise (SNR) and contrast to noise (CNR) compared to 3T when using a turbo-FLASH (TFL) acquisition with an adiabatic spectrally selective RF pre-pulse (SPAIR) for lipid suppression. However, prior to the studies performed at the CMRR, imaging of the left coronary artery (LCA) had not been shown, with the suggested challenge being that poor myocardial-blood contrast would diminish the ability to visualize the vessels. The apparent lack of contrast most likely originates from the absence of sufficient transmit B1 (B1+) in the heart especially over the course of the left coronary artery which is deeper within the body than the right. In general, the contrast between the blood pool and myocardium has been shown to be similar or even greater at higher field strengths. To address the challenge of low peak B1+ and the known challenge of B1+ inhomogeneity at 7T in the context of imaging the LCA, multiple optimized B1+ shimming solutions within a single acquisition sequence were employed using a multi-channel surface array coil. This strategy allowed the LCA to be imaged at 7T with similar contrast to that achieved in the RCA but with lower SNR due to the increased distance of the former from the RF coil. This is the first demonstration of left coronary artery imaging at 7T.
REFERENCES
Metzger GJ, Snyder C, Akgun C, Vaughan T, Ugurbil K, Van de Moortele PF. Local B(1) (+) shimming for prostate imaging with transceiver arrays at 7T based on subject-dependent transmit phase measurements. Magn Reson Med 2008;59(2):396-409.
Snyder CJ, DelaBarre L, Moeller S, Tian J, Akgun C, Van de Moortele PF, Bolan PJ, Ugurbil K, Vaughan JT, Metzger GJ. Comparison Between Eight- and Sixteen-Channel TEM Transceive Arrays for Body Imaging at 7. Magn Reson Med (In Press).
Metzger GJ, van de Moortele PF, Akgun C, Snyder CJ, Moeller S, Strupp J, Andersen P, Shrivastava D, Vaughan T, Ugurbil K, Adriany G. Performance of external and internal coil configurations for prostate investigations at 7 T. Magn Reson Med 2010;64(6):1625-1639.
Metzger GJ, Simonson J, Bi X, Weale P, Zuehlsdorff S, Auerbach EJ, Ugurbil K, Van de Moortele PF. Initial Experiences with Non-Contrast Enhanced Renal Angiography at 7.0 Tesla. Proc Intl Soc Mag Reson Med 2010;18:#403.
Snyder CJ, DelaBarre L, Metzger GJ, van de Moortele PF, Akgun C, Ugurbil K, Vaughan JT. Initial results of cardiac imaging at 7 Tesla. Magn Reson Med 2009;61(3):517-524.
Suttie JJ, Delabarre L, Pitcher A, van de Moortele PF, Dass S, Snyder CJ, Francis JM, Metzger GJ, Weale P, Ugurbil K, Neubauer S, Robson M, Vaughan T. 7 Tesla (T) human cardiovascular magnetic resonance imaging using FLASH and SSFP to assess cardiac function: validation against 1.5 T and 3 T. NMR Biomed 2011.
Metzger GJ, DelaBarre L, Bi X, Shah S, Zuehlsdorff S, Vaughan JT, Ugurbil K, Metzger GJ. Left Coronary Artery Imaging at 7T: Initial Results using Multiple B1+ Shimming Algorithms and Targets. Proc Intl Soc Mag Reson Med 2011;19.