
CMRR
Center for Magnetic Resonance Research, Department of Radiology
FAIR ASST Sequence
You are here
FAIR ASST
Background
The intrinsic low signal-to-noise ratio (SNR) characteristics of arterial spin labeling (ASL) and reduced SNR in slices acquired later in the sequence due to signal losses from T1 relaxation and label washout make whole-brain high-resolution perfusion imaging challenging. High-resolution perfusion imaging can be better achieved by using only limited number of slices sufficiently covering brain regions of interest, such as the hippocampus or the cerebellum. However, when applying the widely used pulsed arterial spin labeling (PASL) method FAIR (Flow-sensitive Alternating Inversion Recovery) for such studies of regions in the mid-brain or lower brain, the superior labeling of FAIR can introduce confounding effects for cerebral blood flow (CBF) quantification and adverse artifacts (1-2). FAIR with Active Suppression of Superior Tagging (FAIR ASST) is a variant of FAIR that resolves those issues. In FAIR ASST, the superior tagging of FAIR is suppressed by applying saturations at the labeling stage on the superior side of the imaging slab. The implementation of FAIR ASST provided in this package uses the mode 12 for superior tagging suppression (2).
Features
The FAIR ASST sequence contains several important and flexible features, allowing further customizations to satisfy users’ specific needs.
- Imaging slab pre-saturation is supported with two execution modes: a single saturation before ASL inversion RF pulses or three saturations using mode 12 as for ASST (1-2). The latter can provide improved imaging slab pre-saturation and minimize the subtraction errors caused by the imperfect inversion profile (3).
- When both pre-saturation and superior saturation are requested, a single saturation RF pulse will be applied covering both the superior tagging region and imaging slab (Figure 1).
- HSN RF pulse (N>= 4) can be selected for FAIR inversions to reduce RF peak power that is usually limited at ultra high fields, e.g. 7T.
- Both QUIPSS II and Q2TIPS are supported with great flexibility via multiple user interface (UI) parameters: the number, duration, slab size and temporal gaps of saturation RF pulses to define temporal bolus width (please refer Figure 1 in the reference 2).
- Up to two M0 images can be acquired at the end of ASL series acquisition.
- Bi-polar flow-encoding/crushing gradients are available to suppress hyperintense intravascular signals.
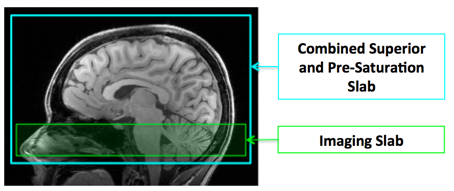
Figure 1. A single large saturation slab (cyan) is used
when both superior and pre-saturation are required.
CMRR C2P
If you are interested in obtaining these sequences, the CMRR C2P instructions and agreement can be found on this page at the UMN Office for Technology Commercialization. Once you have executed a C2P agreement and have been given an access password, the sequence binaries can be downloaded here.
Note to users: If you have noticed a bug or have a request for a new feature in a future release, please email Xiufeng Li lixx1607@umn.edu to open a new support request. Be sure to include the sequence variant and the model of scanner you are using in the problem description.
Download FAIR ASST Sequence:
|
FAIR ASST Release 1.0 |
|
|
Released date FAIR ASST Release Notes v1.0 |
Download coming soon
 Release 1.0 for VB17 (build ) Release 1.0 for VB17 (build ) |
Relevant Patent
Li X, Gopinath K, Sarkar SN, Briggs RW; System and Methods for Active Suppression of Superior Tagging in Flow-Sensitive Alternating Inversion Recovery. United States patent 8,143,892. March 27th, 2012.
References
1. Li X, Sarkar SN, Purdy DE, Haley RW, Briggs RW. Asymmetric FAIR - FAIR with Active Suppression of Superior Tagging (FAIR ASST). In: Proceedings of the 18th Annual Meeting of ISMRM, Stockholm, Sweden 2010:Abstract 1737.
2. Li X, Sarkar SN, Purdy DE, Haley RW, Briggs RW. Improved quantification of brain perfusion using FAIR with active suppression of superior tagging (FAIR ASST). J Magn Reson Imaging. 2011;34(5):1037-1044; doi: 10.1002/ jmri.22734.
3. Li X, Metzger G. Feasibility of Measuring Prostate Perfusion with Arterial Spin Labeling. NMR Biomed. 2013 Jan; 26(1):51-7. doi: 10.1002/nbm.2818.